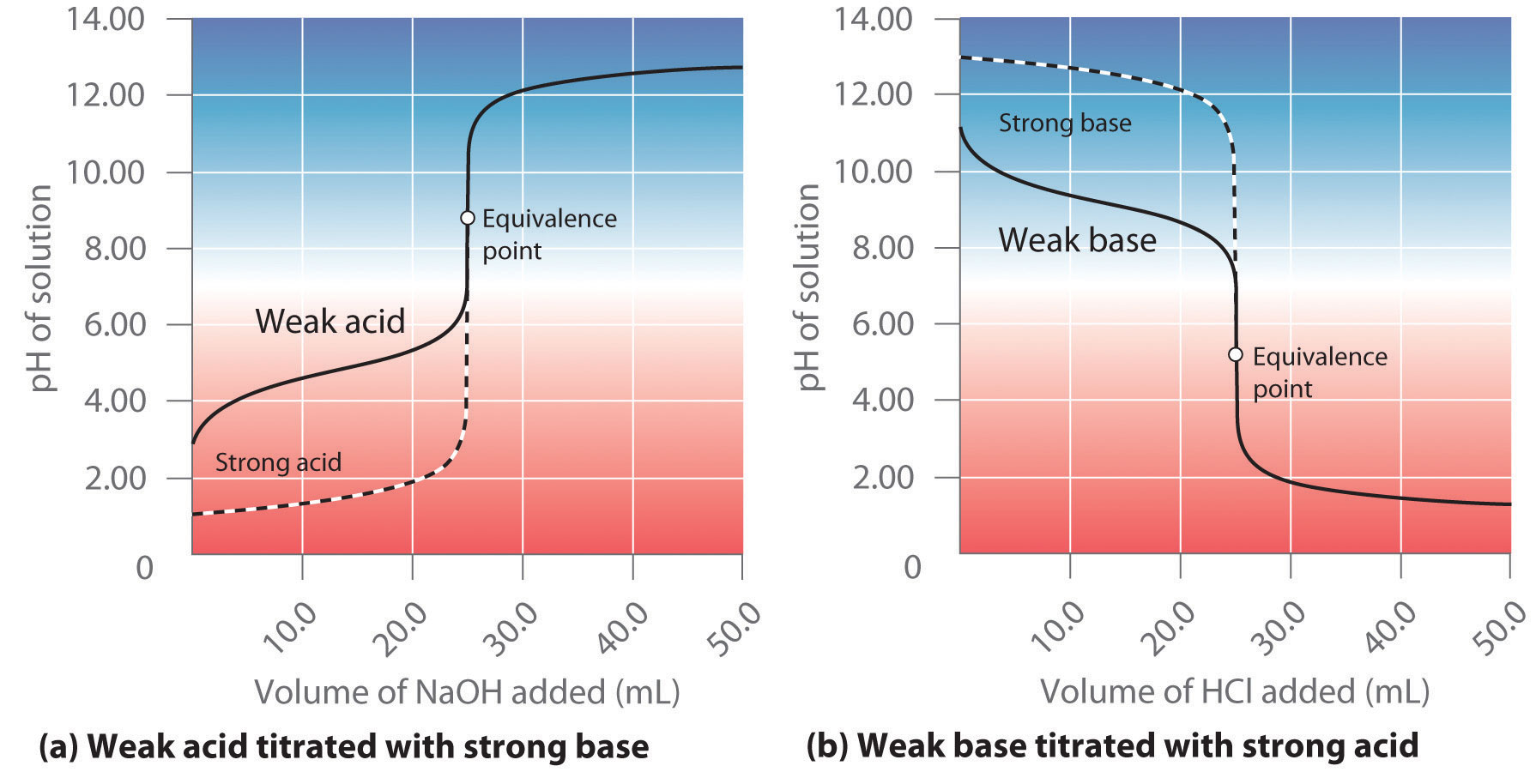
Why Use Titration To Find Concentration . Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Suppose that a titration is performed and \(20.70 \: In many cases it is not a simple matter to obtain a pure substance,. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is often used to determine the concentration of a solution. The basic process involves adding a. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).
from saylordotorg.github.io
Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required to reach the end point when titrated. Titration is often used to determine the concentration of a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. In many cases it is not a simple matter to obtain a pure substance,. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte).
AcidBase Titrations
Why Use Titration To Find Concentration Suppose that a titration is performed and \(20.70 \: In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. \ce{naoh}\) is required to reach the end point when titrated. Titration is often used to determine the concentration of a solution. The basic process involves adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. In many cases it is not a simple matter to obtain a pure substance,. Suppose that a titration is performed and \(20.70 \: Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of.
From socratic.org
How to calculate the concentration of the acid solutions? Socratic Why Use Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. In a titration, a. Why Use Titration To Find Concentration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Why Use Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The basic process involves adding a. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Titration is a quantitative analysis to determine the concentration of an unknown. Why Use Titration To Find Concentration.
From www.microlit.com
An Advanced Guide to Titration Microlit Why Use Titration To Find Concentration A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. In a titration, a solution of known concentration (the titrant) is added to a solution of the substance being studied (the analyte). Titration is often used to determine the concentration of a solution. Suppose that a titration is performed. Why Use Titration To Find Concentration.
From sites.google.com
Chemistry Blog bright's blogs Why Use Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration, process of. Why Use Titration To Find Concentration.
From www.youtube.com
Titration of unknown weak acid with strong base YouTube Why Use Titration To Find Concentration Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. \ce{naoh}\) is required to reach the end point when titrated. Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of known concentration is used. Why Use Titration To Find Concentration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Why Use Titration To Find Concentration Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. In a titration, a solution of known concentration (the titrant) is added to a solution of. Why Use Titration To Find Concentration.
From www.youtube.com
Calcium and Magnesium ion concentration determination with EDTA Why Use Titration To Find Concentration Suppose that a titration is performed and \(20.70 \: Titration is often used to determine the concentration of a solution. The basic process involves adding a. In many cases it is not a simple matter to obtain a pure substance,. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown. Why Use Titration To Find Concentration.
From vasasimonmackenzie.blogspot.com
Equivalence Point Titration Simon Mackenzie Why Use Titration To Find Concentration Suppose that a titration is performed and \(20.70 \: The basic process involves adding a. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. \ce{naoh}\). Why Use Titration To Find Concentration.
From www.slideserve.com
PPT TITRATION PowerPoint Presentation, free download ID1459481 Why Use Titration To Find Concentration Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. Suppose that a titration is performed and \(20.70 \: Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. In many cases it is not a. Why Use Titration To Find Concentration.
From www.science-revision.co.uk
Titrations Why Use Titration To Find Concentration Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is often used to determine the concentration of a solution. Suppose that a titration is performed and \(20.70 \: \ce{naoh}\) is required. Why Use Titration To Find Concentration.
From shelbyscrosbyo.blob.core.windows.net
Titration Method Definition at shelbyscrosbyo blog Why Use Titration To Find Concentration \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. A. Why Use Titration To Find Concentration.
From www.easybiologyclass.com
What is Titration Curve? How Do You Find pKa? easybiologyclass Why Use Titration To Find Concentration In many cases it is not a simple matter to obtain a pure substance,. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is often used to determine the concentration of a. Why Use Titration To Find Concentration.
From saylordotorg.github.io
AcidBase Titrations Why Use Titration To Find Concentration \ce{naoh}\) is required to reach the end point when titrated. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. The basic process involves adding a. A titration is a laboratory technique used. Why Use Titration To Find Concentration.
From www.youtube.com
How to Calculate Concentration using Titration Example in Chemistry Why Use Titration To Find Concentration Suppose that a titration is performed and \(20.70 \: Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. \ce{naoh}\) is required to reach the end point when titrated. In many cases it is not a simple matter to obtain a pure substance,. Titration, process of chemical analysis in which the quantity of some. Why Use Titration To Find Concentration.
From www.slideserve.com
PPT Calculating concentrations PowerPoint Presentation, free download Why Use Titration To Find Concentration A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. The basic process involves adding a. Suppose that a titration is performed. Why Use Titration To Find Concentration.
From chem.libretexts.org
Complexation Titration Chemistry LibreTexts Why Use Titration To Find Concentration Suppose that a titration is performed and \(20.70 \: A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. Titration is often used to determine the concentration of a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding. Why Use Titration To Find Concentration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Why Use Titration To Find Concentration Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by adding to the measured sample an exactly known quantity of. In many cases it is not a simple matter to obtain a pure substance,. The basic process involves adding a. In a titration, a solution of known concentration (the titrant) is added. Why Use Titration To Find Concentration.
From courses.lumenlearning.com
AcidBase Titrations General Chemistry Why Use Titration To Find Concentration Titration is a quantitative analysis to determine the concentration of an unknown solution by adding a. \ce{naoh}\) is required to reach the end point when titrated. The basic process involves adding a. A titration is a technique where a solution of known concentration is used to determine the concentration of an unknown solution. A titration is a laboratory technique used. Why Use Titration To Find Concentration.